最近几十年以来,荧光聚合物受到越来越多的关注与研究[1-4]。荧光聚合物相对于荧光小分子来说,具有易成型加工、结构可设计性强、多功能化等诸多优点[1,3]。其中荧光聚氨酯(PU)是最具有应用前景的聚合物之一,因其结构与性能的多样性使其不仅在纺织、涂料、皮革等传统领域扮演着重要角色[5],也在显示[6-7]、温度识别[8]、生物成像[9]、形状记忆[9-10]、挥发性有机物(VOC)检测[11]等新兴智能应用领域崭露头角。
荧光聚氨酯是通过将染料分子以化学键形式引入大分子链上得到的,Li等[12]通过自制几种含有蒽醌发色团的水溶性染料单体,并将其作为扩链剂进行高速分散乳化,制备出共价着色的PU胶乳,提供了一种制备荧光聚氨酯的通用方法。但是,对于染料共价键合聚氨酯的荧光性质研究,目前都偏重于乳液体系,对于荧光聚氨酯的固态荧光发射性质研究甚少。如:Hu等[13]将染料4–氨基–N–环己基–1,8–萘二甲酰亚胺(ACN)通过预聚物–离聚物过程键合到聚氨酯分子链上,研究了乳液的温度、溶剂等因素以及乳胶颗粒和猝灭剂[14]对乳液荧光性质影响。Wang等[15]也是将一系列染料键合到聚氨酯分子链上制得阴离子型荧光聚氨酯胶粘剂,重点研究了其耐碱性[16]及其对皮革材料的染色性能[17]。Jin等[18]虽然研究了PU固态薄膜,但是侧重于其耐溶剂性能和耐光性能,对其固态荧光发射性质没有研究。而具有固态发射的荧光聚合物在OLED显示、化学传感器、生物探针、细胞成像等高新技术应用领域内是不可或缺的[19-22]。因此,在染料键合型荧光聚氨酯领域,其固态荧光发射性质还亟待研究。
1,5–二羟基萘是一种染料中间体,稀溶液状态下可以发射荧光,但在固体状态下由于其分子含有萘环,易发生平面Ⅱ–Ⅱ堆积作用而导致聚集荧光猝灭(aggregation-caused quenching,ACQ)。如果将其通过化学键形式引入聚氨酯分子链中,借助大分子的分子间相互作用和分子链空间缠绕阻碍限制1,5–二羟基萘平面Ⅱ–Ⅱ堆积作用,就可制得一种新型的荧光聚氨酯,使其在固态下也将有荧光发射。另外,得益于分子中氨基和荧光基团的光致电子转移(photoinduced electron transfer,PET)过程,新型荧光聚氨酯还将有酸碱响应荧光行为,可作为酸碱响应型的智能材料。作者采用溶液聚合法,分预反应与聚合反应两步,将1,5–二羟基萘作为荧光功能单体引入聚氨酯分子链中,制备出了一种新型的多功能荧光聚氨酯(MFU)。系统表征了其分子结构,重点研究了MFU的荧光发光性质和酸碱响应行为。
1 实验部分 1.1 实验原料4,4′–二环己基甲烷二异氰酸酯(HMDI)、1,4–丁二醇(BDO)、二月桂酸二丁锡(DBTDL)和聚丁二醇(PTMG,Mn = 1000 g/mol),山东万华化学集团有限公司,分析纯;1,5–二羟基萘(1,5–DN),Adamas公司,试剂纯;N,N–二甲基甲酰胺(DMF)、无水乙醇,成都科隆化工有限公司,分析纯;PTMG在真空度0.1 MPa、温度110 ℃条件下脱水处理3 h待用;DMF在使用前先用4A分子筛干燥48 h。
1.2 合成方法称取HMDI 7.87 g(0.03 mol),定量1,5–DN (具体质量见表1,溶于DMF溶剂),加入3滴催化剂DBTDL,先在80 ℃下N2气氛中进行2 h预反应,得到含有功能基团的二异氰酸酯。通过滴定法监控NCO含量,结果与理论值一致,证明1,5–DN已完全反应。再加入真空脱水的PTMG 10.00 g(0.01 mol) 聚合反应3 h,最后加入定量扩链剂BDO (具体质量见表1)再反应3 h进一步提高聚氨酯分子量。反应结束后,用两倍体积的去离子水沉析提纯后于真空烘箱干燥即得到MFU样品。改变1,5–二羟基萘荧光单体在聚氨酯分子链中的质量占比,合成不同荧光单体含量的MFU样品,编号为0~4。合成路线见图1,具体配方见表1。将MFU样品溶解在无水乙醇中获得溶液样品。将MFU样品溶解于DMF(固含量10%),浇在四氟乙烯培养皿中,用真空烘箱缓慢烘干溶剂得到薄膜样品。
| 表1 不同含量MFU样品的配方 Tab. 1 Recipes of MFU samples with different contents |
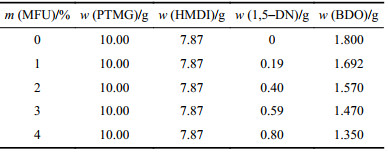 |
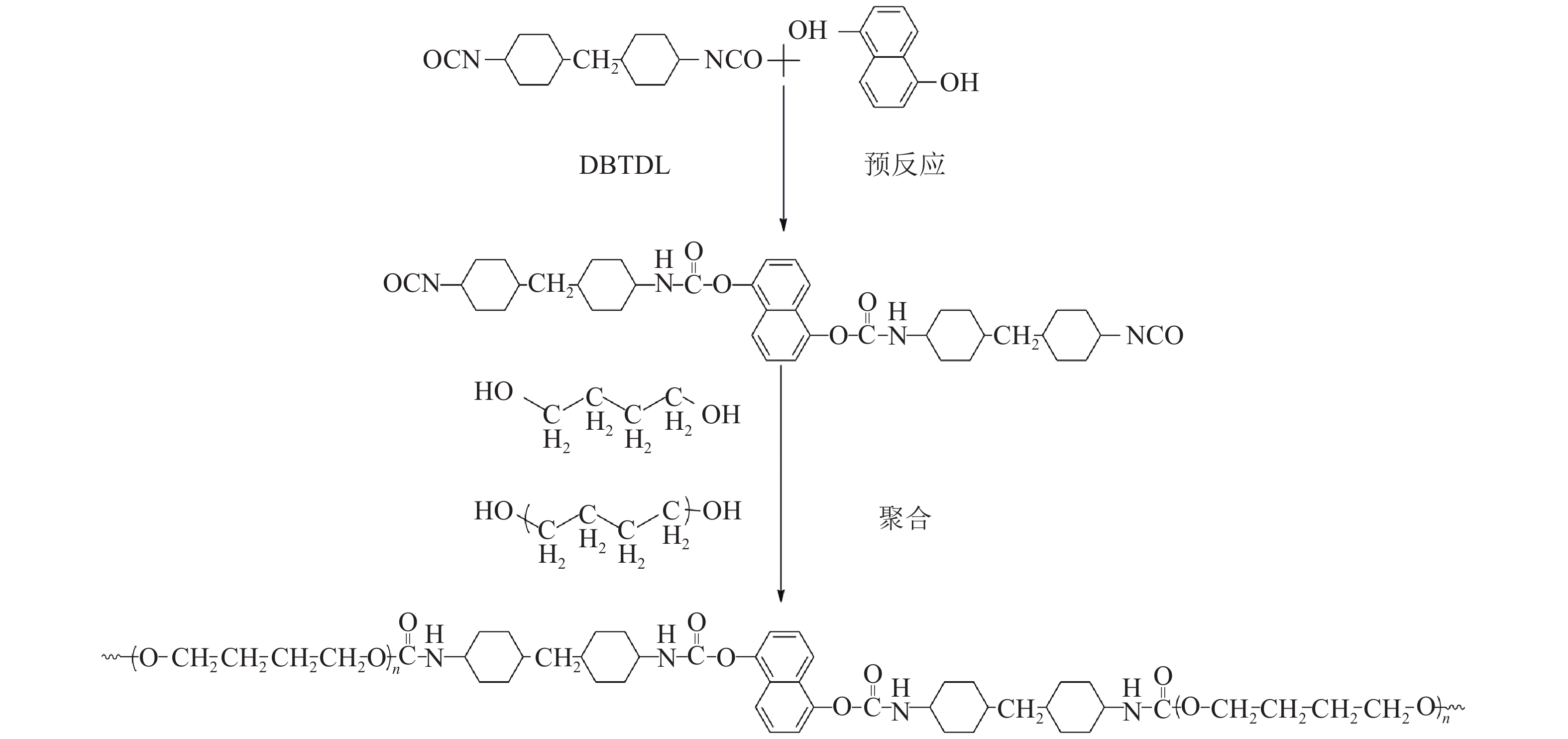 |
| 图1 荧光聚氨酯合成路线 Fig. 1 Synthesis route of fluorescent polyurethane |
1.3 表征方法
核磁氢谱(1H NMR)测试:样品溶解于氘代二甲基亚砜(DMSO–d6)中,以四甲基硅烷(TMS)为内标物,在AVII–600核磁共振仪(Bruker,瑞士)上表征,扫描64次。
紫外–可见光谱(UV–vis)测试:MFU样品配置成浓度为4×10–5 g/mL的无水乙醇溶液,室温下在UV–3600(岛津,日本)分光光度计上记录了紫外–可见光谱;采用浓度8.0×10–7、1.6×10–6、4.0×10–6、5.0×10–6g/mL 功能单体1,5–二羟基萘的无水乙醇溶液为标准溶液做出标准曲线。
红外测试(IR):样品溶解于DMF溶剂中,四氟乙烯培养皿中烘干成膜。薄膜样品在具有全反射(ATR)的傅里叶变换红外分光光度计(thermo fisher scientific,美国)中采集红外光谱,扫描频率范围为4000~800 cm–1。
荧光光谱(FL)测试:在Fluoromax–4荧光光谱仪(Horiba,日本)上记录荧光光谱。溶液样品为浓度4.0×10–5 g/mL的无水乙醇溶液。
2 结果与讨论 2.1 结构表征图2为PU和MFU(4%)的1H NMR谱图。由图2可知:δ=7.2~7.6范围内,萘的质子峰出现在荧光聚氨酯(MFU 4%)样品中,而纯聚氨酯样品谱图中没有出现相应质子峰,表明萘进入了聚氨酯分子链中。δ=7.96处为聚氨酯分子链硬段中N—H质子峰。δ=3.32是软段聚醚中与氧相连的—CH2的质子峰。另外,化学位移δ=1.74、1.52、1.48和1.04是HMDI脂环上的质子峰以及软段中非氧相连的—CH2质子峰。
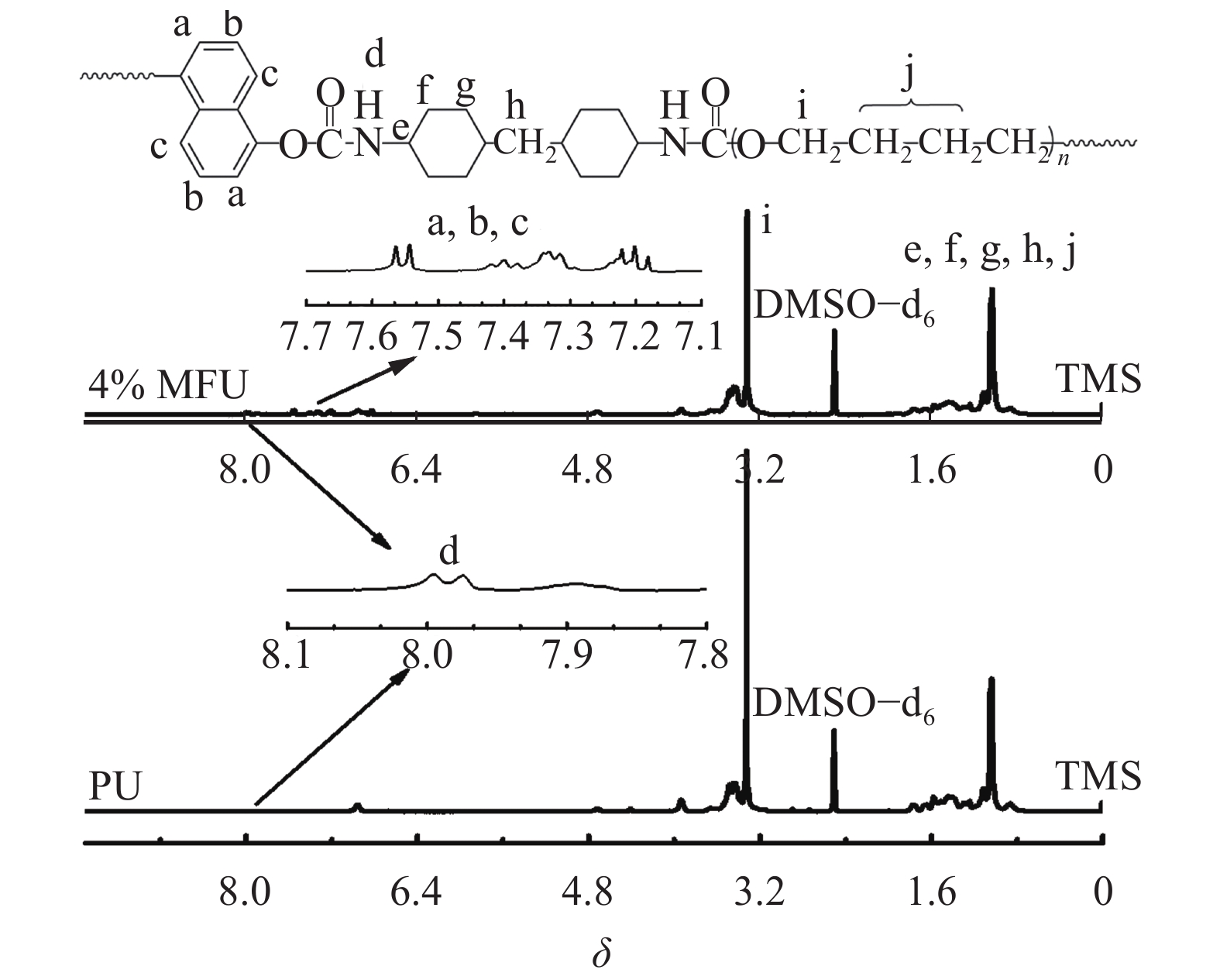 |
| 图2 PU和MFU(4%)的1H NMR谱图 Fig. 2 1H NMR spectrum of PU and MFU (4%) |
图3为PU和MFU(1%~4%)的红外光谱。由图3可知:N—H的伸缩振动峰出现在3322 cm–1左右,分子链中饱和CH2的伸缩振动峰在2969、2924和2854 cm–1。在1730 cm–1处出现氨基甲酸酯中C=O的特征吸收峰,在N—H的影响下略微向低波数移动了。在1243、1227 cm–1处出现软段中C—O—C伸缩振动峰。在1630、1548、1483 cm–1左右出现萘环呼吸振动峰,其中4%含量的样品在1630 cm–1的峰最明显。1548 cm–1处出现强吸收峰为N—H的面外弯曲振动峰,在MFU样品中其与萘环的呼吸峰重合。在2260 cm–1处无峰,表明—NCO反应完全。
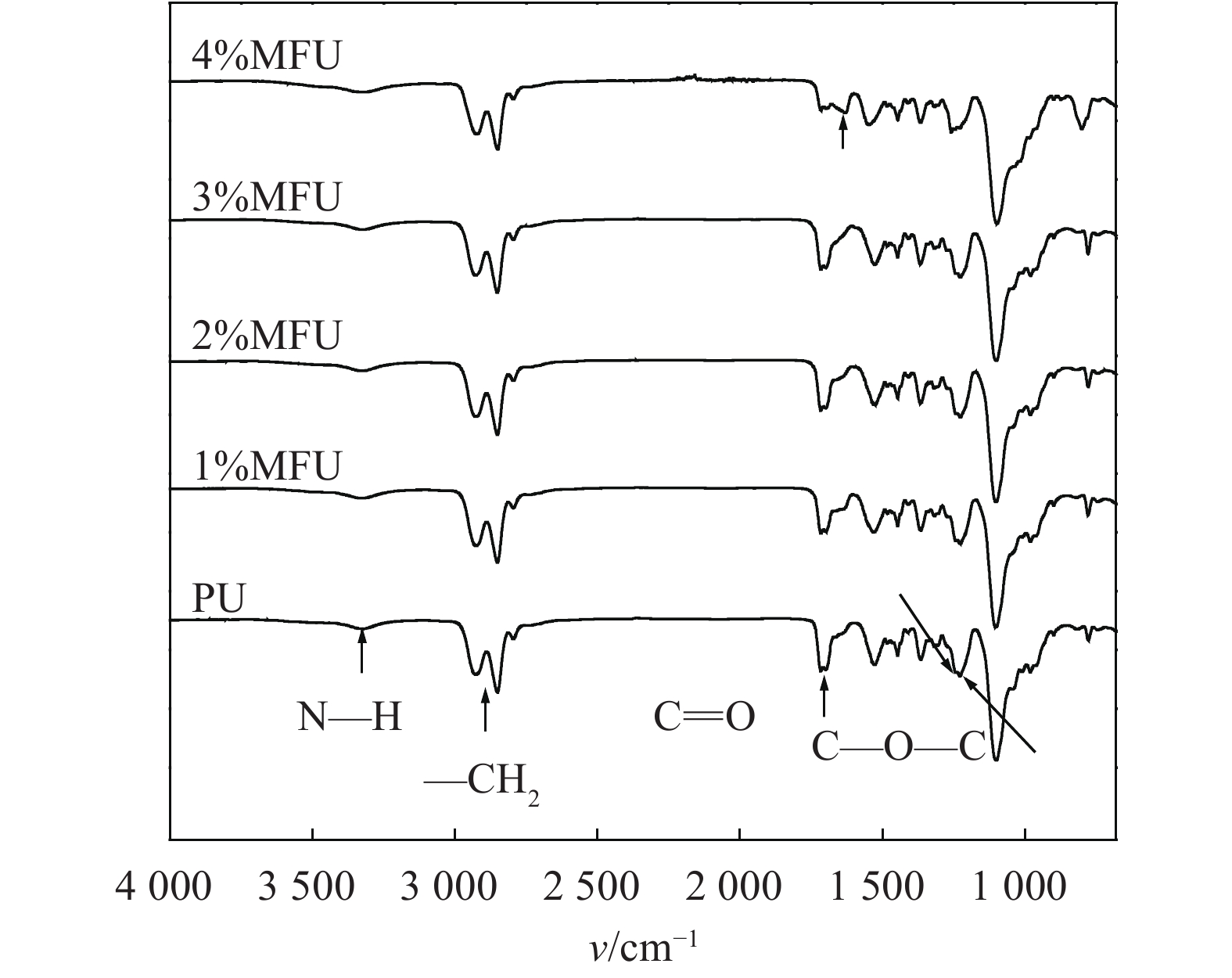 |
| 图3 纯聚氨酯和MFU(1%~4%)样品的红外光谱 Fig. 3 IR spectrum of pure polyurethane and MFU (1%~4%) |
图4为纯聚氨酯、MFU(1%~4%)和1,5–二羟基萘的无水乙醇溶液的紫外吸收光谱,图5为不同浓度的1,5–二羟基萘单体的无水乙醇溶液紫外吸收光谱。
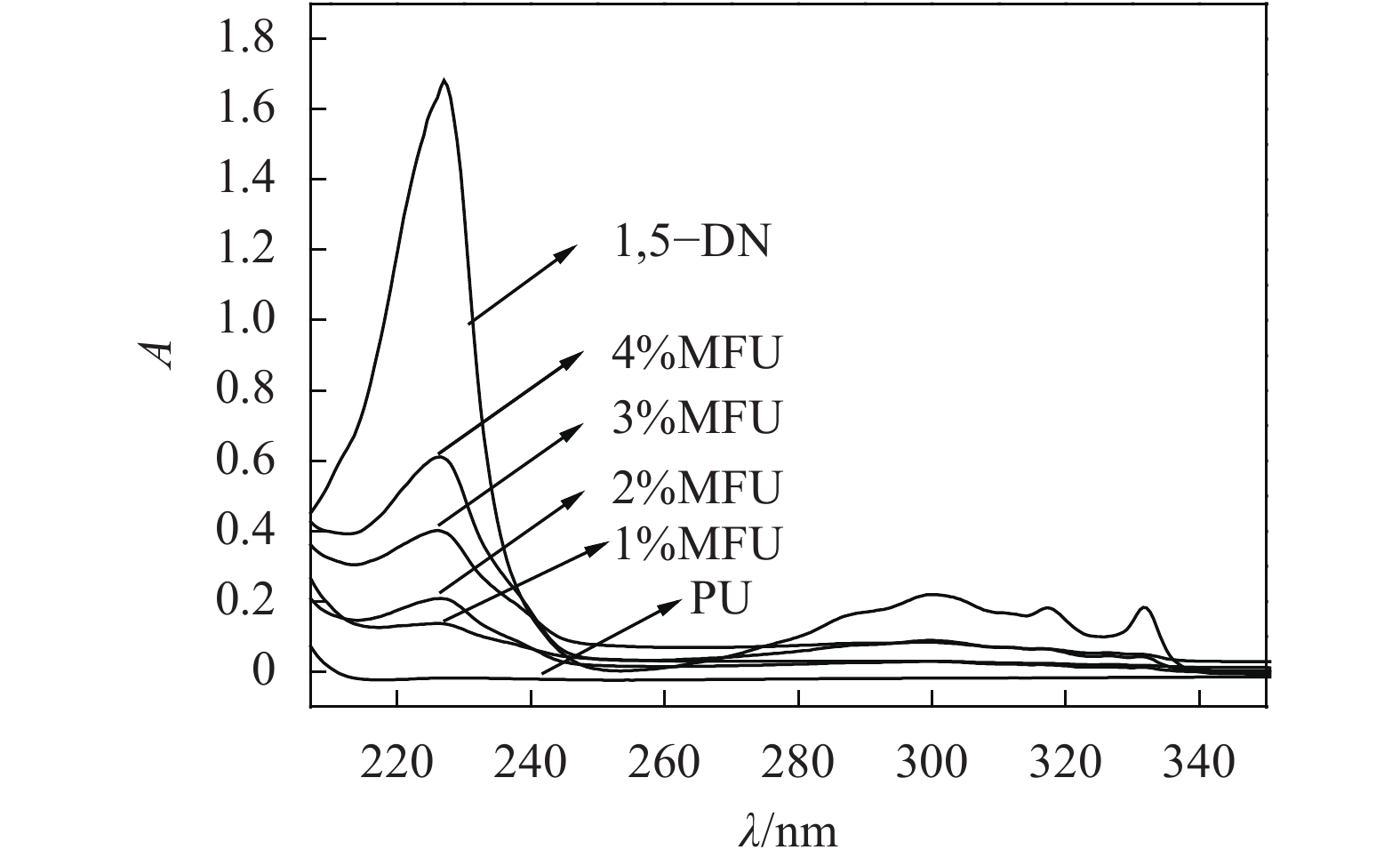 |
| 图4 PU和MFU(1%~4%)样品的紫外吸收光谱 Fig. 4 UV absorption spectra of PU and MFU (1%~4%) samples |
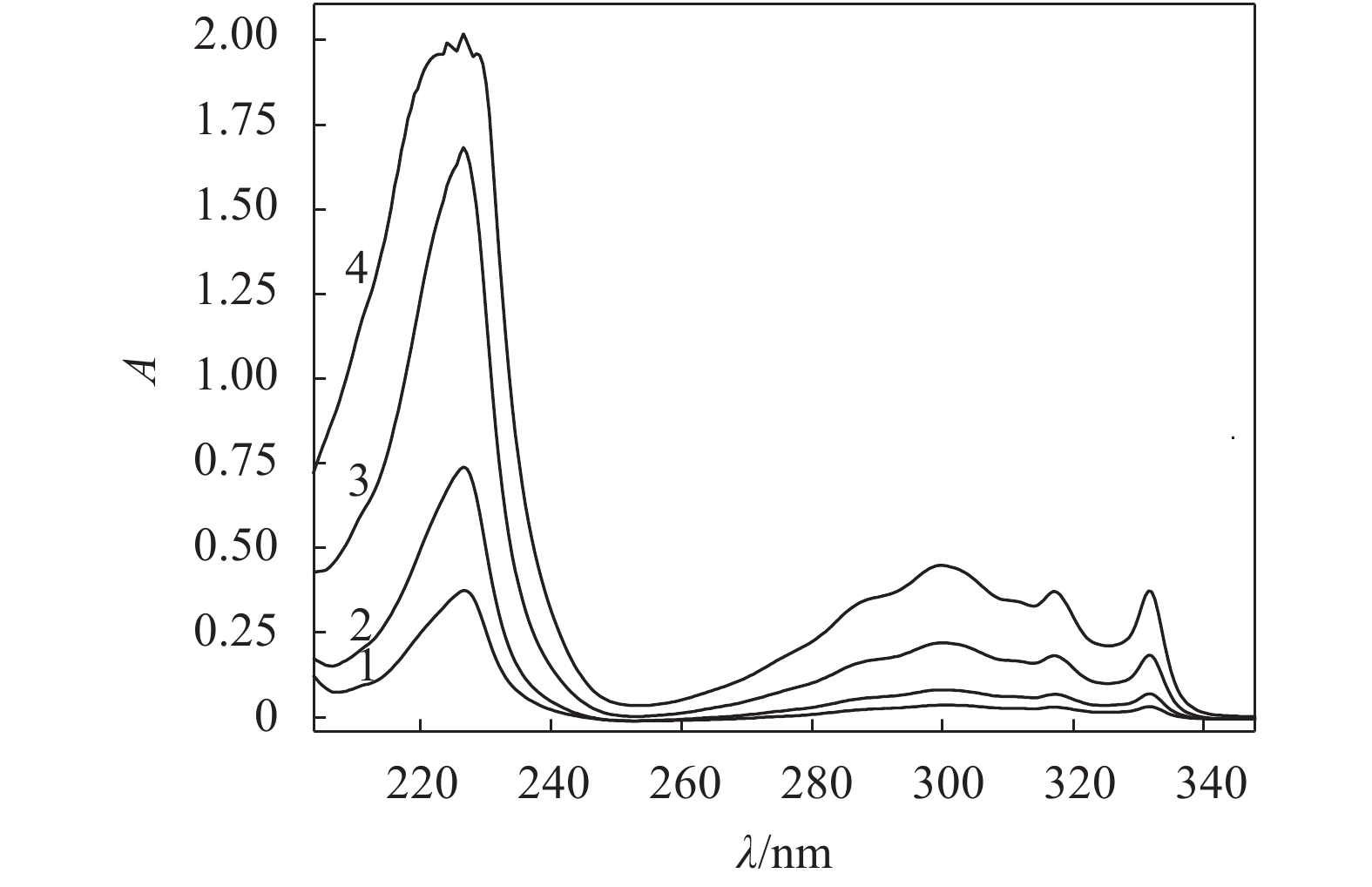 |
| 样品1、2、3、4浓度依次为8×10–7、1.6×10–6、4×10–6、5×10–6 g/mL。 图5 1,5–二羟基萘单体溶液的紫外吸收光谱以及标准曲线 Fig. 5 UV absorption spectra and standard curves of solution of 1,5–dihydroxy naphthalene monomer |
由图4可知,1,5–二羟基萘单体与MFU(1%~4%)溶液样品吸收主要集中在213~340 nm近紫外区,而纯聚氨酯在此区间没有明显吸收。MFU样品由于分子链中含有萘环基团,于226 nm处出现萘环特征强吸收峰,且随着羟基萘含量增加紫外吸收强度上升,这归属于萘环基团共轭体系的π→π*跃迁[23]。另外,还在260~340 nm范围内也出现了萘环的吸收谱带[24],为B吸收带,由苯环振动与π→π*跃迁重叠引起[25]。MFU样品的两个吸收带与1,5–二羟基萘单体的吸收谱带分布吻合。这也再一次证明,羟基萘单体进入了聚氨酯分子链上,与核磁光谱、红外光谱结果一致。
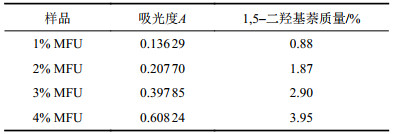
另外,根据朗伯–比尔定律,配制不同浓度的1,5–二羟基萘单体标准溶液分别在相同条件下测试其紫外–可见光谱(图5),以226 nm处的特征强吸收峰为基准,将吸光度对浓度拟合成过原点的直线,作为标准曲线(y=68481.2138x,R2=0.9991),可以估算出MFU样品分子链中引入萘环基团的含量(质量占比)[26-27]。液层厚度取1 cm,相关计算参数列于表2。由表2可知,随着1,5–二羟基萘在MFU分子链中含量增加,吸收强度逐渐增强,经紫外定量计算表明,误差允许范围内,MFU分子链中实际的1,5–二羟基萘含量与理论质量比一致。
| 表2 MFU样品的吸光度与1,5–二羟基萘质量百分含量 Tab. 2 Absorbance and mass percentage of 1,5–dihydroxynaphthalene of MFU samples |
 |
2.2 MFU的荧光发射性质 2.2.1 固态荧光效应
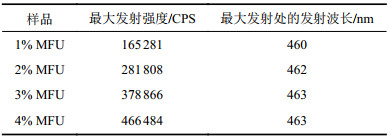
图6为不同1,5–二羟基萘含量的MFU薄膜样品以及1,5–二羟基萘单体粉末(1,5–DN)的荧光光谱。图6显示,MFU固体膜样品相对纯聚氨酯对照来说,在400~550 nm宽范围内具有较强荧光发射,而荧光单体1,5–二羟基萘固体粉末在紫外激发下,几乎不发光,呈现典型的聚集发射猝灭现象(ACQ)。这是因为,固体聚集状态下,萘环平面接触紧密,形成Ⅱ–Ⅱ堆积,被激发时高能量分子平面与低能量分子平面发生能量转换,无需辐射跃迁即将能量耗散掉,所以导致发光猝灭[19-20]。而将荧光单体1,5–二羟基萘以化学键形式键入聚氨酯大分子链主链后,由于大分子链的空间缠绕阻碍作用,使得荧光基团良好的分散在聚氨酯中而不发生荧光猝灭。根据图6将最大荧光发射强度和在最大发射处的发光波长列于表3。由表3可知,其最大发射峰处的发光波长在460 nm左右且略有红移。
| 表3 MFU薄膜样品的最大发射强度和最大发射处的发射波长 Tab. 3 Maximum emission intensity and emission wavelength at maximum emission of MFU film samples |
 |
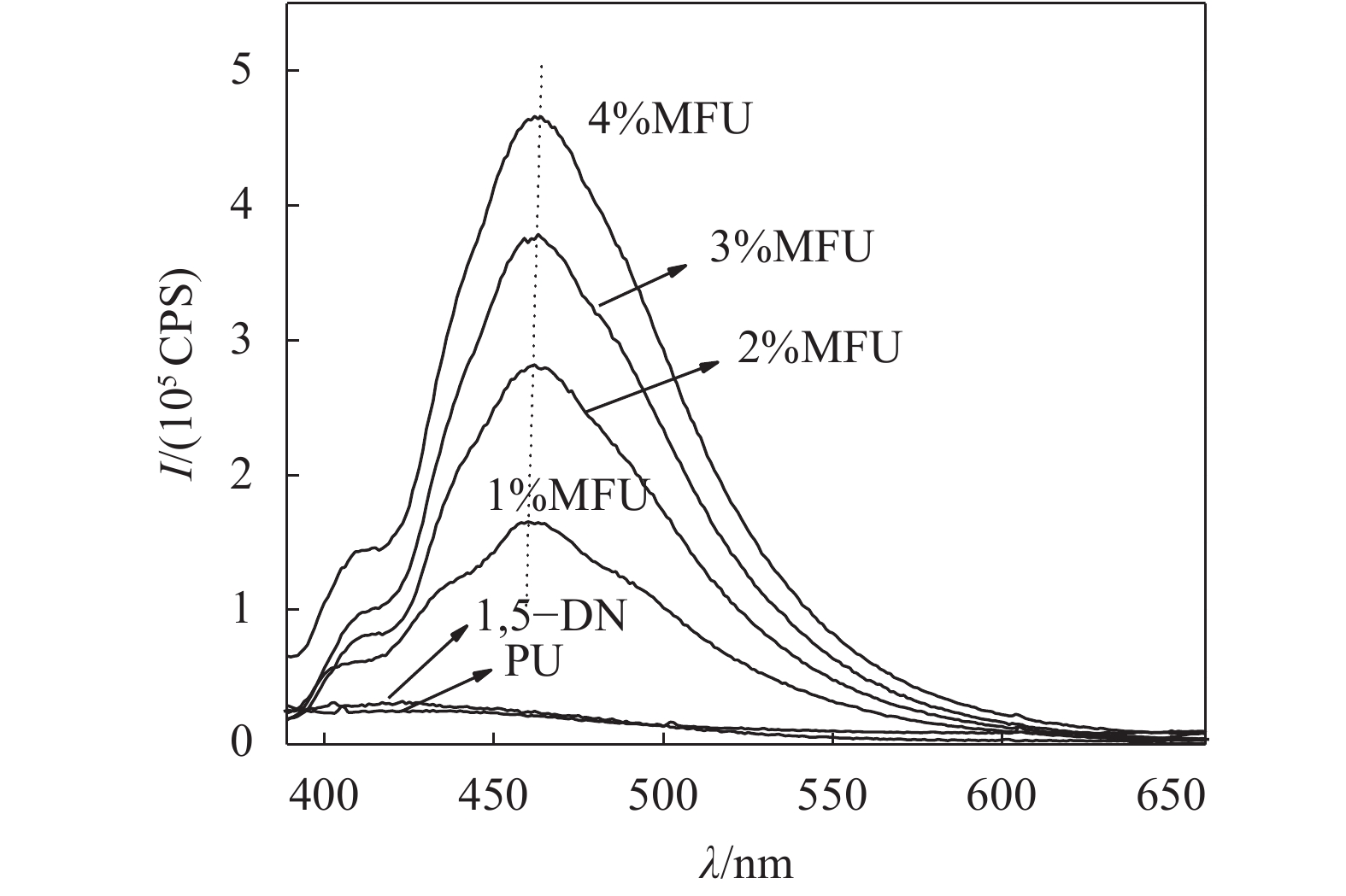 |
| 图6 纯聚氨酯、MFU(1%~4%)和1,5–DN的固态荧光光谱 Fig. 6 Solid fluorescence spectra of pure polyurethane, MFU (1%~4%) and 1,5–DN |
2.2.2 溶液荧光效应
图7为纯聚氨酯、MFU(1%~4%)和1,5–DN的溶液荧光光谱,可以看出,纯聚氨酯样品没有明显的荧光发射峰,而1,5–二羟基萘稀溶液和MFU样品具有明显的荧光发射峰,且MFU样品随着1,5–DN含量增加,荧光发射强度增大。根据荧光光谱,将MFU溶液样品的最大发射强度和最大发射处的发射波长参数列于表4。
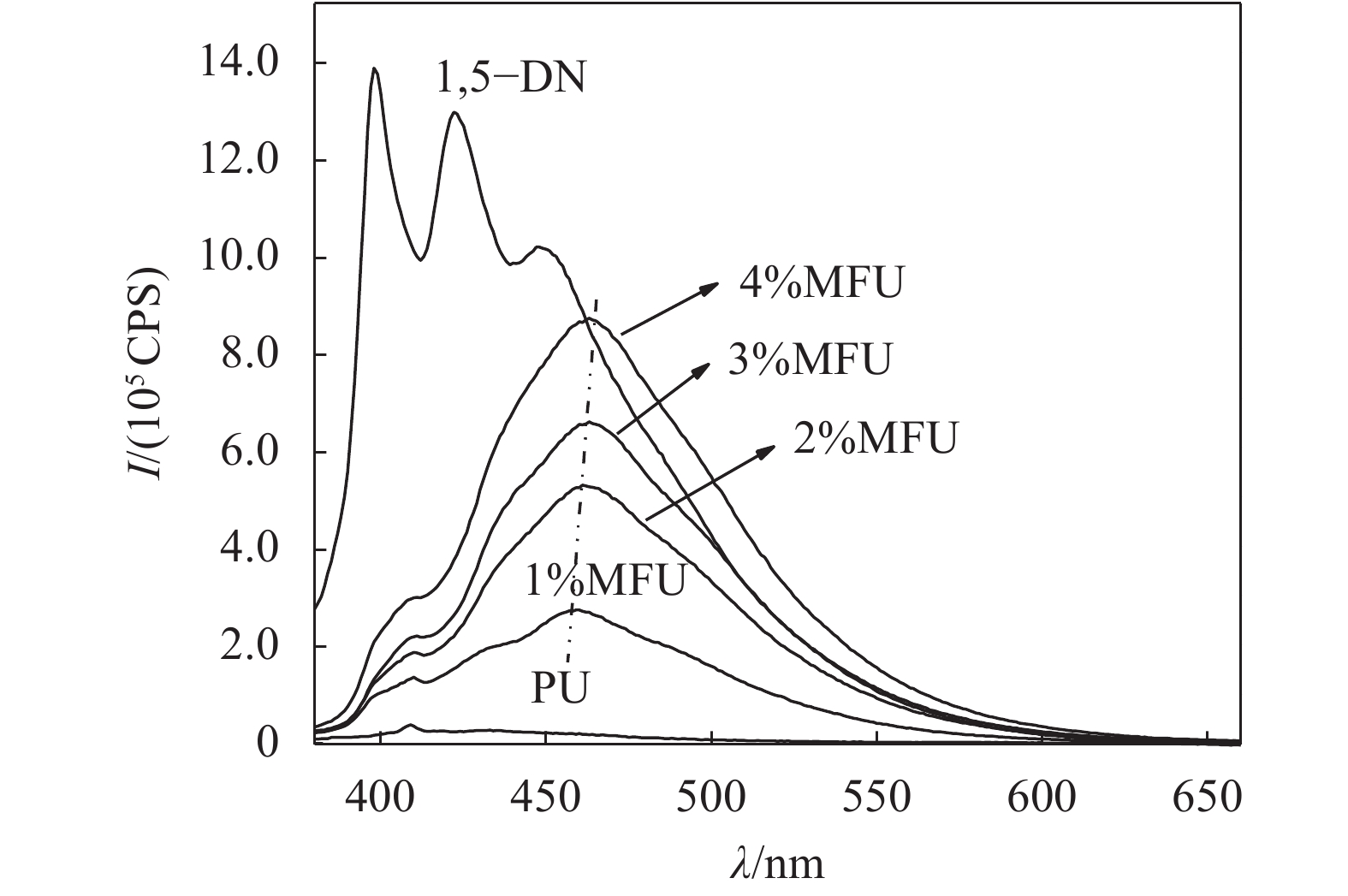 |
| 溶液浓度为4×10–5 g/mL,激发波长为365 nm。 图7 纯聚氨酯,MFU(1%~4%)和1,5–DN的溶液荧光光谱 Fig. 7 Solution fluorescence spectra of pure polyurethane, MFU (1%~4%) and 1,5–DN |
| 表4 MFU溶液样品的最大发射强度和最大发射处的发射波长 Tab. 4 Maximum emission intensity and emission wavelength at maximum emission of MFU solution samples |
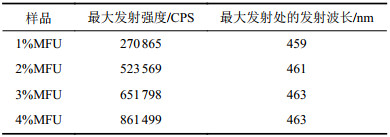 |
由表4可知,4%MFU样品的荧光强度相对于1%MFU样品的荧光强度提升了218%,说明微量荧光单体的增加即可大幅度提高MFU样品的荧光强度,这得益于荧光基团在聚合物中以单分散状态而不是堆积聚集状态存在。1,5–二羟基萘与MFU样品的荧光光谱对比可知,MFU荧光发射峰明显红移,这是因为羟基萘引入聚氨酯大分子链后,萘环受周围氨基甲酸酯键影响,电子离域程度增大,降低了π→π*电子跃迁能量,去激发过程也相应能量降低,故发生红移[28-29]。
2.2.3 MFU的酸碱响应行为MFU样品在酸碱(酸为盐酸,碱为氢氧化钠)不同环境下,呈现不一样的荧光发光性质如图8所示(所有样品浓度均为4×10–5 g/mL,激发波长均为365 nm)。由图8可知:碱性条件下,最大荧光发发射峰波长在460 nm左右(蓝光);而酸性条件下最大荧光发射峰波长在490 nm(蓝绿光)左右,明显发生红移,位移30 nm。
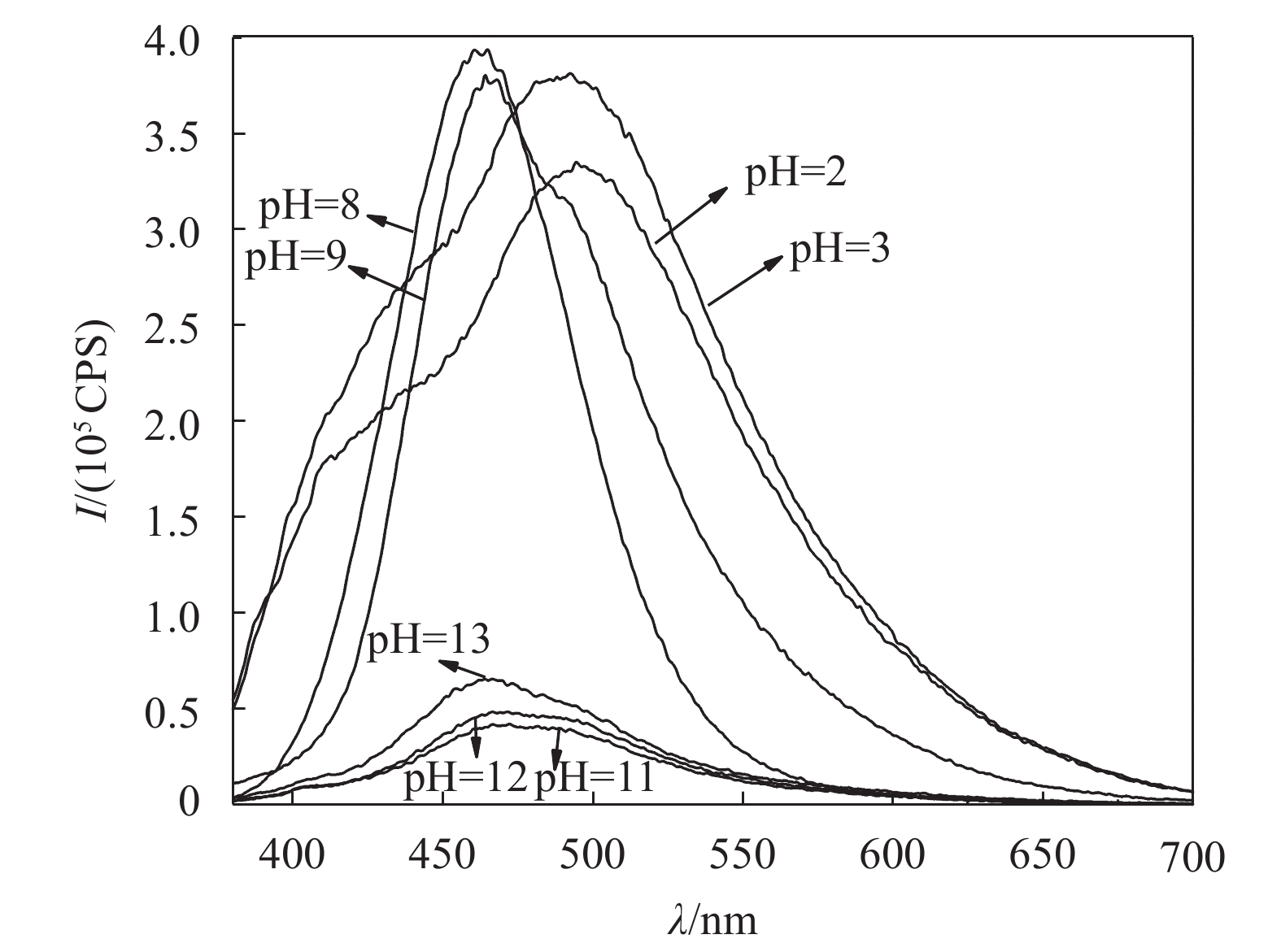 |
| 图8 MFU溶液样品在不同pH环境下的荧光光谱 Fig. 8 Fluorescence spectra of MFU solution samples under different pH conditions |
另外,在荧光发射强度上,强碱(PH>11)条件下的荧光发射强度相对于强酸性和弱碱性条件的荧光发射强度发生数量级的下降,如图9所示。由图9可知,PH=11条件下的荧光强度比PH=3时的荧光强度下降了89%。这是由于在酸性条件下,致使亚氨基被质子化,减弱了光诱导电子转移(PET)过程,使得荧光发射增强[30-33]。
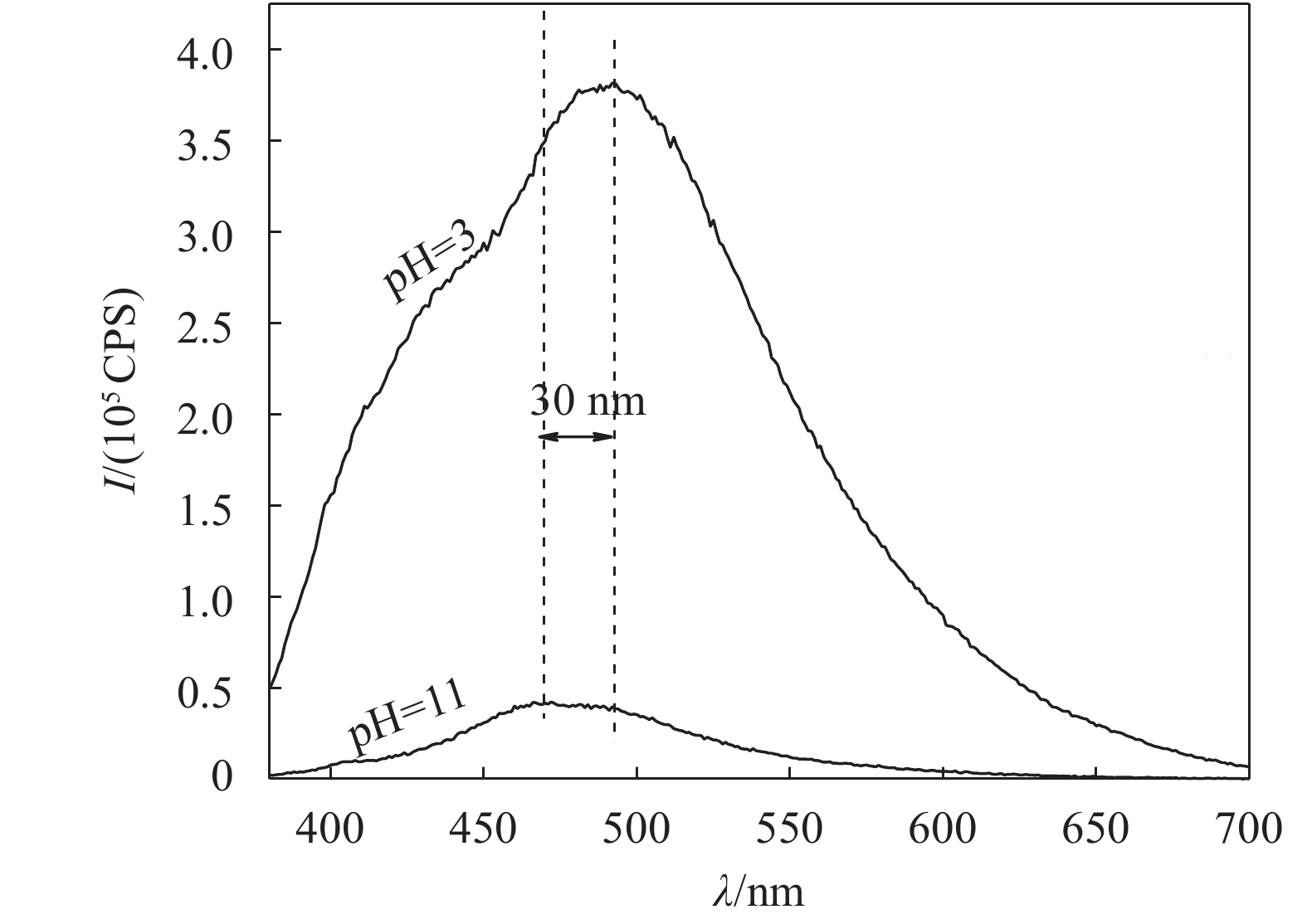 |
| 图9 强酸(pH=3)和强碱(pH=11)环境下的荧光光谱 Fig. 9 Fluorescence spectra of the two samples under strong acid (pH=3) and strong base (pH=11) conditions |
加入酸使得发射增强,是因为在紫外光激发下,萘环基团的电子从最高占据分子轨道跃迁(HOMO,激发后变为S0)被激发到更高的能量单重态(S1、S2等)。被激发的电子经历某种程度的内部转换或无辐射去激励过程,通常将能量分配到周围的介质中,然后在转换回基态时发射光子和荧光。由于亚氨基的存在,酸性环境下,发生PET过程,常规激发–去激发过程被中断。亚氨基(PET供体)上的一对孤电子在能量上占据萘环荧光团S0和S1轨道之间的轨道(图10)。被激发至S1的电子从该轨道转移到荧光团的S0轨道(图10)。于是,萘环荧光团的基态被完全占据,S1中的激发态电子放宽到亚氨基的单个占据的轨道上,而不是萘环荧光团的S0轨道上。这两个较低的能量跃迁回避了荧光跃迁,猝灭了发射[30,34]。
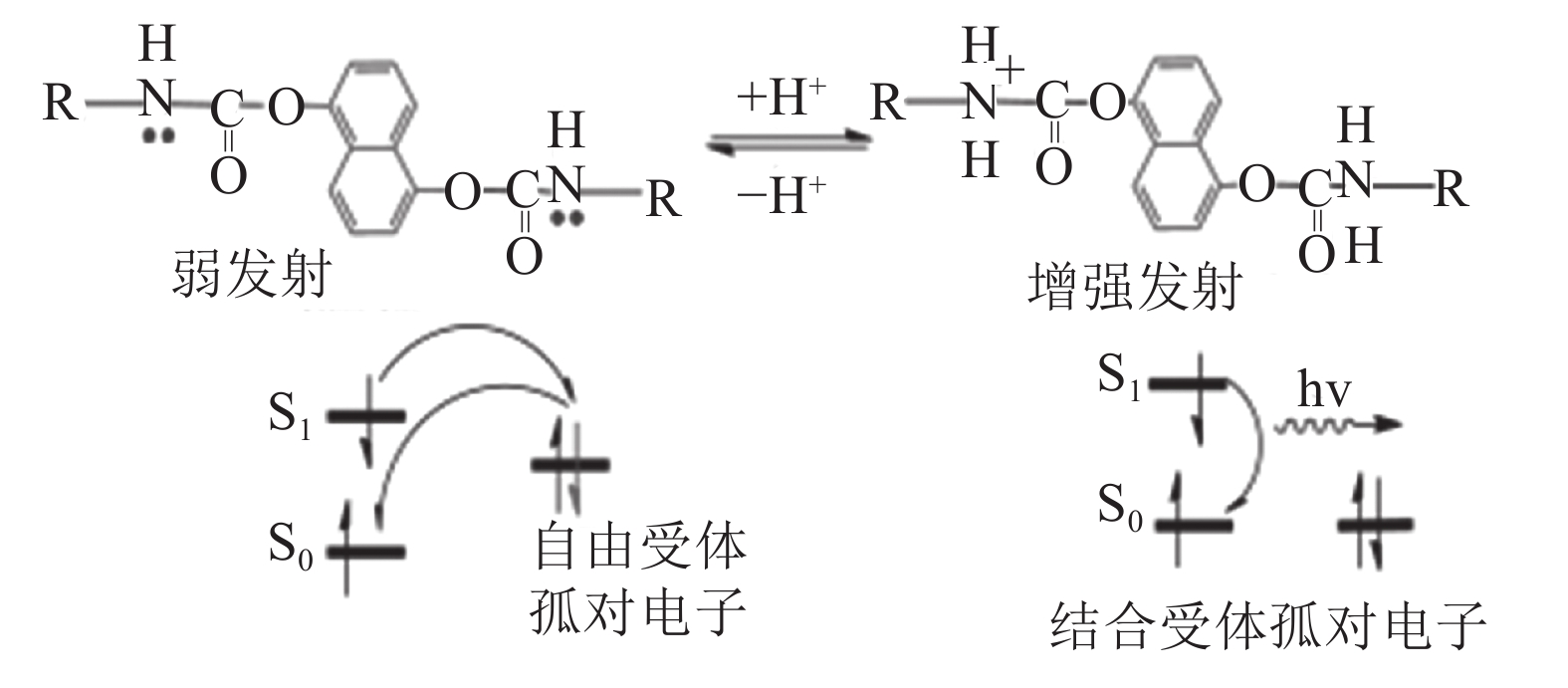 |
| 图10 MFU的PET机理 Fig. 10 PET mechanism of MFU |
基于MFU样品在强酸强碱环境下,表现出的发光颜色与发光强度的差异,即双特征响应行为,有望被用于强酸强碱的双信号探针。
3 结 论将1,5–二羟基萘作为荧光功能单元以化学键接方式引入聚氨酯分子链中,成功合成了多功能荧光聚氨酯(MFU),其同时具有固态和酸碱响应荧光发射性质。固体状态下,由于聚氨酯大分子链的缠绕阻碍作用,消除了荧光聚集猝灭现象,致使MFU在固体状态下也能有荧光发射。另外,MFU溶液样品在对酸碱环境表现出荧光响应行为,在强碱(pH>11)和强酸(pH<3)环境下,具有发光颜色和发光强度双重变化特征,有望用于强酸和强碱环境下的双信号探测。
| [1] |
Gui R,Jin H. Recent advances in synthetic methods and applications of photo-luminescent molecularly imprinted polymers[J]. Journal of Photochemistry and Photobiology(C):Photochemistry Reviews, 2019, 41: 100315. DOI:10.1016/j.jphotochemrev.2019.08.002 |
| [2] |
Kim H N,Guo Z,Zhu W,et al. Recent progress on polymer-based fluorescent and colorimetric chemosensors[J]. Chemical Society Reviews, 2011, 40(1): 79-93. DOI:10.1039/C0CS00058B |
| [3] |
Liu Z,Jiarui W,Wang C,et al. Stimuli-responsive fluorescent supramolecular polymer network based on a monofunctionalized leaning tower[6]arene[J]. Chinese Chemical Letters, 2019, 30(12): 2299-2303. DOI:10.1016/j.cclet.2019.10.023 |
| [4] |
Thomas S W,Joly G D,Swager T M. Chemical sensors based on amplifying fluorescent conjugated polymers[J]. Chemical Reviews, 2007, 107(4): 1339-1386. DOI:10.1021/cr0501339 |
| [5] |
Chattopadhyay D K,Raju K V S N. Structural engineering of polyurethane coatings for high performance applications[J]. Progress in Polymer Science, 2007, 32(3): 352-418. DOI:10.1016/j.progpolymsci.2006.05.003 |
| [6] |
Janietz S,Kruger H,Wedel A,et al.Polyurethanes with covalent attached fluorescent dyes as deep red emitting materials[C]//Proceedings of Organic Light-Emitting Materials and Devices,2002.Seattle:WA:238–247.
|
| [7] |
Gao L,Li C,Wang C,et al. Structure and luminescent property of polyurethane bonded with Eu3+ complex
[J]. Journal of Luminescence, 2019, 212: 328-333. DOI:10.1016/j.jlumin.2019.02.055 |
| [8] |
Ji X,Zhang W,Ge F,et al. Thermochromic behavior analysis of terminated polyurethane functionalized with rhodamine B derivative[J]. Progress in Organic Coatings, 2019, 131: 111-118. DOI:10.1016/j.porgcoat.2019.02.022 |
| [9] |
Gopinath A,Subaraja M,Sultan Nasar A. Fluorescent shape-memory hyperbranched polyurethanes:Synthesis,characterization and evaluation of cytotoxicity[J]. European Polymer Journal, 2018, 108: 517-528. DOI:10.1016/j.eurpolymj.2018.09.037 |
| [10] |
Chung Y C,Yang K,Choi J W,et al. Characterisation and application of polyurethane copolymers grafted with photoluminescent dyes[J]. Coloration Technology, 2014, 130(4): 305-313. DOI:10.1111/cote.12097 |
| [11] |
Kumar R,Yadav R,Kolhe M A,et al. 8–Hydroxypyrene–1,3,6–trisulfonic acid trisodium salt (HPTS) based high fluorescent,pH stimuli waterborne polyurethane coatings[J]. Polymer, 2017, 136: 157-165. DOI:10.1016/j.polymer.2017.12.056 |
| [12] |
Li B,Shao W,Wang Y,et al. Facile synthesis and characterization of covalently colored polyurethane latex based on the chain extension of water-soluble dye monomer[J]. Progress in Organic Coatings, 2019, 129: 140-146. DOI:10.1016/j.porgcoat.2018.12.027 |
| [13] |
Hu X,Zhang X,Liu J,et al. Synthesis,characterization and fluorescence performance of a waterborne polyurethane-based fluorescent dye 4–amino–N–cyclohexyl–1,8–naphthalimide,WPU–ACN[J]. Polymer International, 2014, 63(3): 453-458. DOI:10.1002/pi.4523 |
| [14] |
Xianhai H,Zhang X,Liu J,et al. Synthesis,characterization and fluorescence performance of a waterborne polyurethane-based polymeric dye[J]. Journal of Luminescence, 2013, 142: 23-27. DOI:10.1016/j.jlumin.2013.02.048 |
| [15] |
Wang H H,Gen C T. Synthesis of anionic water-borne polyurethane with the covalent bond of a reactive dye[J]. Journal of Applied Polymer Science, 2002, 84(4): 797-805. DOI:10.1002/app.10336 |
| [16] |
Wang H H,Yimilzun Lin. Silicon-containing anionic water-borne polyurethane with covalently bonded reactive dye[J]. Journal of Applied Polymer Science, 2003, 90(8): 2045-2052. DOI:10.1002/app.12797 |
| [17] |
Wang H H,Tzai G M,Chang C C. Alkali reduction and reactive dye dyeing of T/N nonwoven fabrics dipped into silicon-containing,water-borne polyurethane[J]. Journal of Applied Polymer Science, 2005, 96(6): 2324-2335. DOI:10.1002/app.21489 |
| [18] |
Jin Q,Li B,Zhang H,et al. Investigation of covalently colored polyurethane latexes based on novel anthraquinone polyurethane chain extenders[J]. Journal of Macromolecular Science(Part A), 2016, 54(1): 52-59. DOI:10.1080/10601325.2017.1250317 |
| [19] |
Hong Y,Lam J W Y,Tang B Z. Aggregation-induced emission:Phenomenon,mechanism and applications[J]. Chemical Communications, 2009(29): 4332-4353. DOI:10.1002/chin.200945263 |
| [20] |
Hong Y,Lam J W Y,Tang B Z. Aggregation-induced emission[J]. Chemical Society Reviews, 2011, 40(11): 5361-5388. DOI:10.1039/C1CS15113D |
| [21] |
Mei J,Hong Y,Lam J W Y,et al. Aggregation-induced emission:The whole is more brilliant than the parts[J]. Advanced Materials, 2014, 26(31): 5429-5479. DOI:10.1002/adma.201401356 |
| [22] |
Mei J,Leung N L C,Kwok R T K,et al. Aggregation-induced Emission:Together we shine,united we soar[J]. Chemical Reviews, 2015, 115(21): 11718-11940. DOI:10.1021/acs.chemrev.5b00263 |
| [23] |
Li Heng,Ren Tianhui,Zhong Qinghua,et al. Synthesis and characterization of novel blue light emitting 9,10–di(naphthalen–2–yl) anthracene derivatives[J]. Liquid Crystals and Displays, 2008, 23(5): 540-544. [李恒,任天辉,钟庆华,等. 新型蓝光材料9,10–二萘蒽衍生物的合成及表征[J]. 液晶与显示, 2008, 23(5): 540-544. DOI:10.3969/j.issn.1007-2780.2008.05.004] |
| [24] |
Coggeshall N D,Glessmer A S. Ultraviolet absorption analysis for naphthalenes[J]. Analytical Chemistry, 1949, 21(5): 550-553. DOI:10.1021/ac60029a005 |
| [25] |
Xie Aijuan,Luo Shiping,Wu Weizhong. Ultraviolet and fluorescence analysis of including naphthalene fluorescence monomer and polymer[J]. Spectroscopy Laboratory, 2012, 29(3): 1609-1614. [谢爱娟,罗士平,吴卫忠. 含萘荧光单体和聚合物的紫外和荧光分析[J]. 光谱实验室, 2012, 29(3): 1609-1614. DOI:10.3969/j.issn.1004-8138.2012.03.076] |
| [26] |
Jin Jing,Liu Yuhang,Yang Hongyu,et al. Synthesis and characterization of fluorescent poly(ethylene glycol)–Polyurethane with carboxyl groups[J]. Polymer Materials Science & Engineering, 2014, 30(5): 11-15. [金晶,刘宇航,杨洪雨,等. 带有羧基基团荧光聚氨酯材料的合成与表征[J]. 高分子材料科学与工程, 2014, 30(5): 11-15.] |
| [27] |
Tong Weifang,Yang Jian,Wu Zhaoqiang,et al. Synthesis and characterization of fluorescent polymers containing naphthalimide moieties for Pd2+ detection
[J]. Materials Review, 2012, 26(5): 39-41. [童伟芳,杨坚,武照强,等. 含萘酰亚胺荧光聚合物的合成及对Pd2+识别研究
[J]. 材料导报, 2012, 26(5): 39-41. DOI:10.3969/j.issn.1005-023X.2012.10.012] |
| [28] |
Li F,Chen S,Li Z,et al. Vinyl monomers bearing chromophore moieties and their polymers I:Initiation and photochemical behavior of N–acryloyl–N′–phenylpiperazines and their polymers[J]. Journal of Polymer Science(Part A Polymer Chemistry), 1996, 34(10): 1881-1888. DOI:10.1002/(SICI)1099-0518(19960730)34:10%3c1881::AID-POLA5%3e3.0.CO;2-S |
| [29] |
Ma Y,Tang Q Y,Zhu J,et al. Fluorescent and thermal properties of siloxane-polyurethanes based on 1,8–naphthalimide[J]. Chinese Chemical Letters, 2014, 25(5): 680-686. DOI:10.1016/j.cclet.2014.01.048 |
| [30] |
Hutt J T,Aron Z D. Efficient,single-step access to imidazo[1,5–a]pyridine N–heterocyclic carbene precursors[J]. Organic Letters, 2011, 13(19): 5256-5259. DOI:10.1021/ol202134n |
| [31] |
Chao D,Yang Y,Jia X,et al. Rationally-designed multi responsive fluorescent switching polymer films[J]. Dyes and Pigments, 2019, 167: 77-82. DOI:10.1016/j.dyepig.2019.04.013 |
| [32] |
Deibler K,Basu P. Continuing Issues with Lead:Recent advances in detection[J]. European Journal of Inorganic Chemistry, 2013, 2013(7): 1086-1096. DOI:10.1002/ejic.201200997 |
| [33] |
Xu D,Liu X,Lu R,et al. New dendritic gelator bearing carbazole in each branching unit:Selected response to fluoride ion in gel phase[J]. Organic & Biomolecular Chemistry, 2011, 9(5): 1523-1528. DOI:10.1039/C0OB00786B |
| [34] |
Hutt J T,Aron Z D. Synthesis and application of ratiometric and “turn-on” fluorescent pH sensors:An advanced organic undergraduate laboratory[J]. Journal of Chemical Education, 2014, 91(11): 1990-1994. DOI:10.1021/ed4006166 |
 2021, Vol. 53
2021, Vol. 53


